中央醫療以科技重塑脊椎健康
中央醫療器材股份有限公司,由林智一先生創立於民國76年(西元1987),主要致力於脊椎醫療器材之設計、研發、生產及銷售。主要產品為脊椎植入醫材、手術器械,以及骨科相關之醫療器材。 中央醫療著力於脊椎疾病治療領域,結合微創手術的思維,發展出一套獨步亞洲並適合亞洲人使用之鈦合金植入器。

產品設計與研發

組裝製造

品質控管與測試驗證

包裝、滅菌與出貨管理
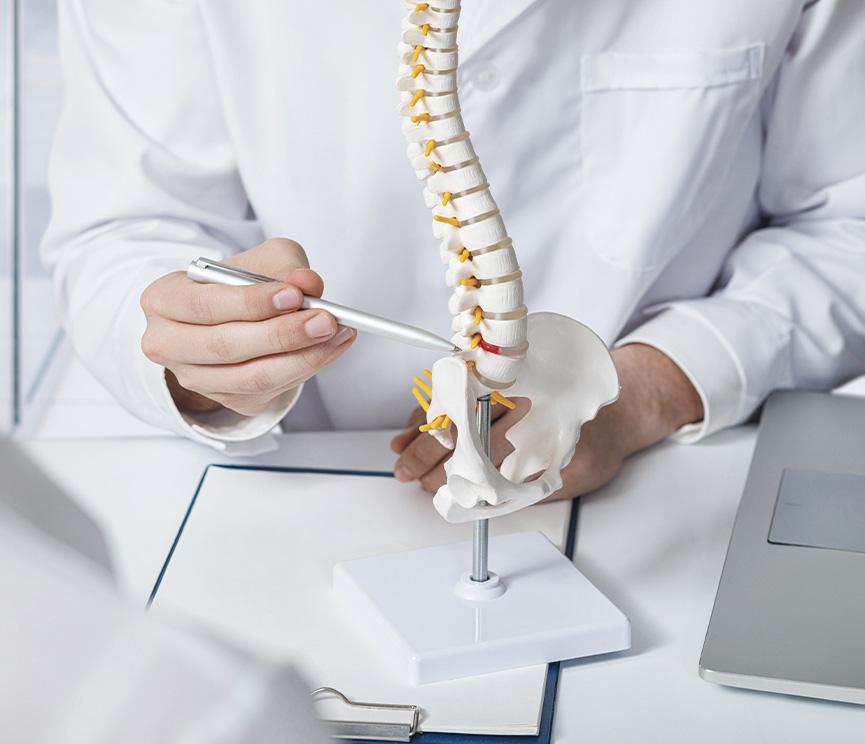
應用領域